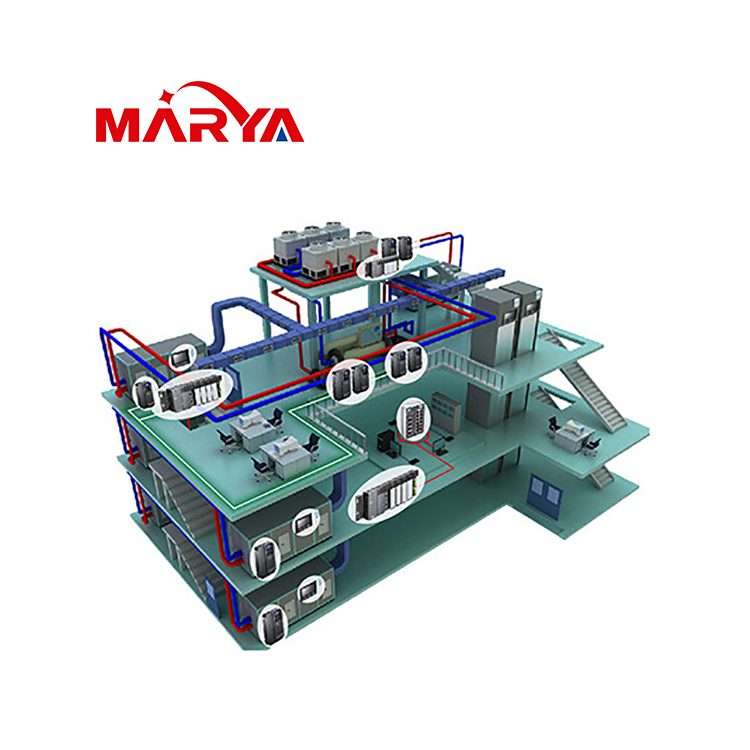
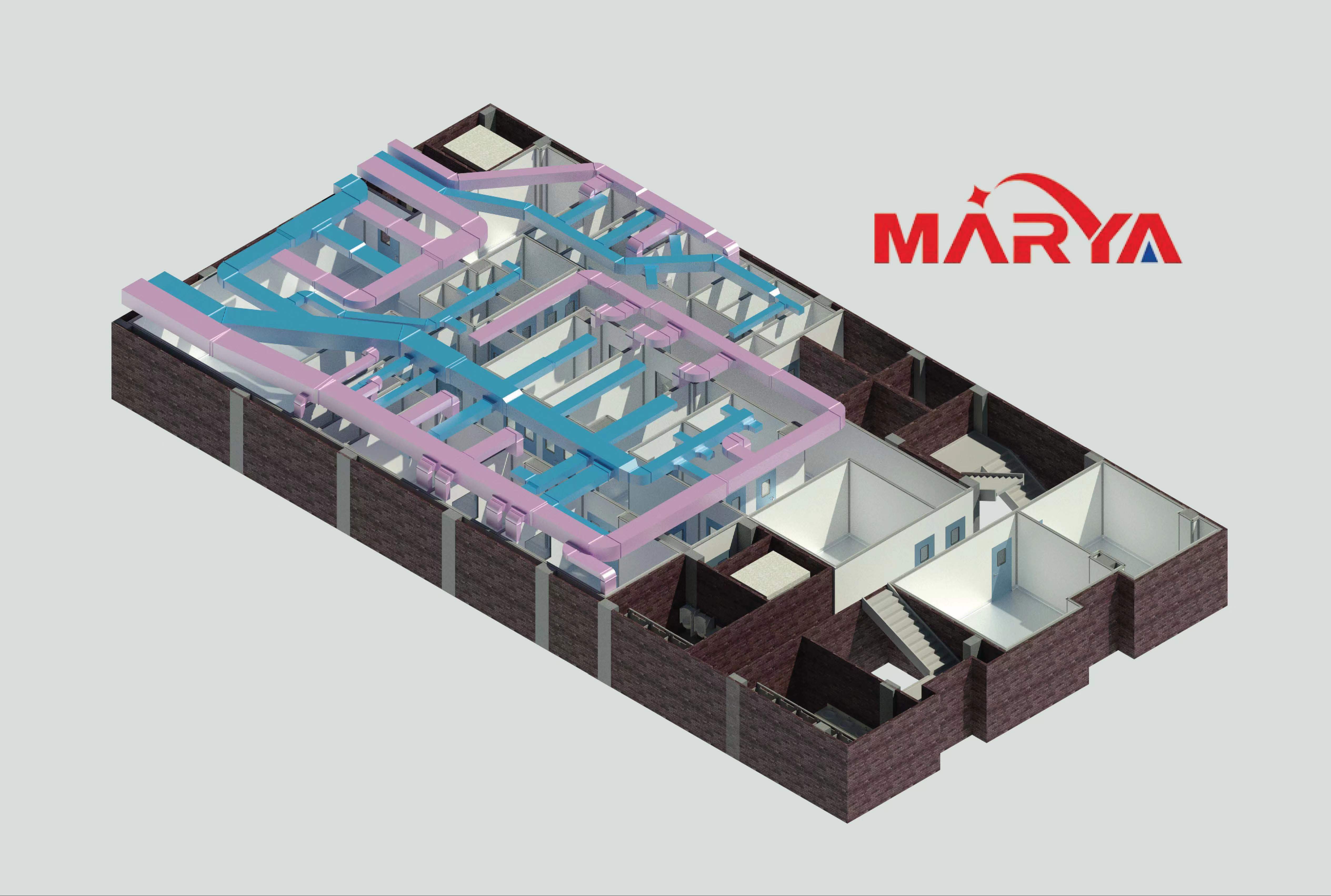
1. Reasonable design air purification system Reasonable design and establishment of clean workshops and effective management are very important in pharmaceutical production. Through the desig...
With the rapid development of modern industry, the technical content of its products is more complex. In order to adapt to more and more industries relying on the production and assembly of their o...
The cleanliness level applicable pharmaceutical production dosage form and process ClassA/ClassB 1) Filling of terminally sterilized large-volume injections (≥50 ml); 2) Non-t...
Compared with the various methods of disinfection and sterilization of the air, and the air cleaning technology using barrier filter equipment, there are the following disadvantages (1) Most methods ...
【Abstract】According to the spirit of GMP, the quality of drugs is not detected by the final drug inspection, but actually produced. Therefore, GMP focuses on the whole process of production, from c...
The purpose of the self-cleaning performance test is to determine the ability and rate of the cleanroom or clean facility to remove suspended particulate pollutants, which is the most important per...
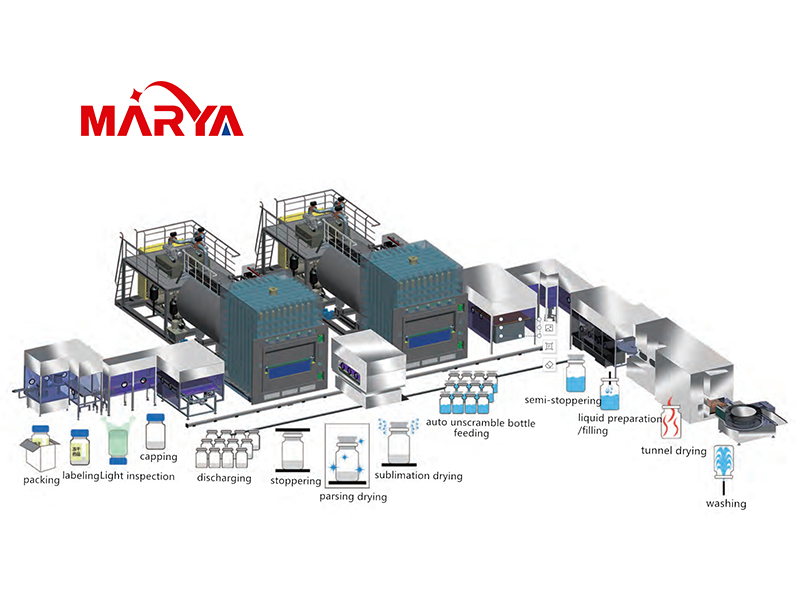
Vacuum freeze drying is also called sublimation drying. The principle is to freeze the material so that the water it contains turns into ice, and then sublimate the ice in a vacuum to dry it. Vacuum ...
Vial is the main packaging form of sterile powder injection drugs now, and the use is large. At the same time, vacuum freeze drying technology has developed very rapidly in recent years, after near...
There are extremely strict requirements for the layout of the flow channels and logistics channels in the cleanroom. That is to say, the entry and exit of people and things must follow prescribed proc...
After the selection of the air purification system is completed, the installation of the purification system is followed. In the process of construction and installation, compared with the hidden w...
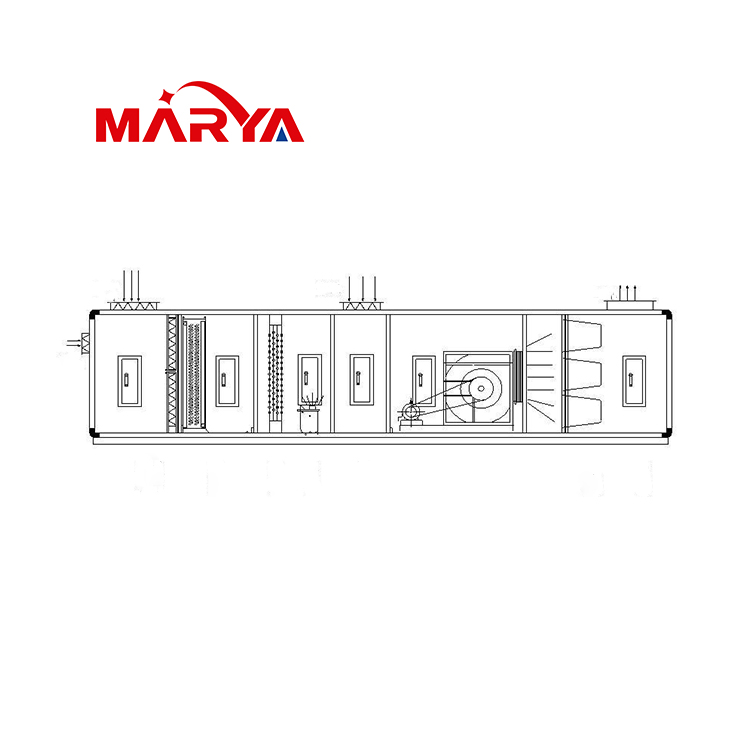
After the installation location of the HVAC system is determined, the selection work of the AHU begins. For a new general contracting project, the design institute may have included all the selecti...
After the vaccine is freeze-dried in the freeze-drying box, it generally needs to be sealed with vacuum or nitrogen filling to prevent the vaccine freeze-dried preparation from being contaminated and ...